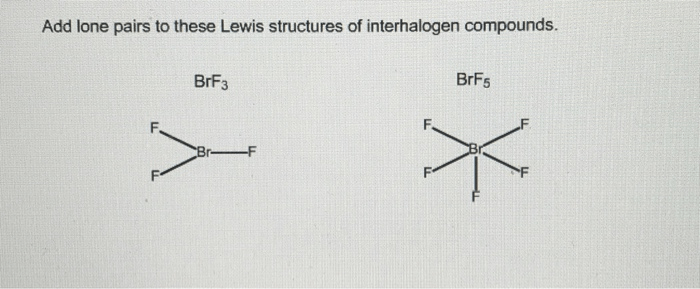
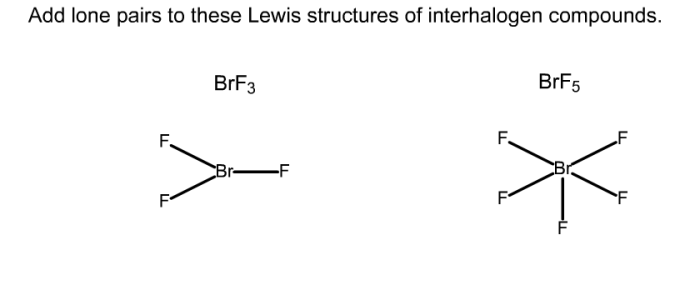
Add lone pairs to these lewis structures of interhalogen compounds. – In the realm of chemistry, understanding the structure and bonding of molecules is crucial. Lewis structures, a powerful tool for representing molecular structures, provide a concise and informative depiction of electron distribution. This article delves into the concept of adding lone pairs to Lewis structures of interhalogen compounds, a crucial step in accurately representing their chemical bonding and behavior.
Interhalogen compounds, formed between two different halogens, exhibit unique properties and reactivity patterns. Adding lone pairs to their Lewis structures allows us to satisfy the octet rule, a fundamental principle in chemistry, and gain insights into their electronic configurations. By exploring the significance of lone pairs and the process of adding them to Lewis structures, we enhance our understanding of these compounds and their role in various chemical reactions.
Lewis Structures of Interhalogen Compounds: Add Lone Pairs To These Lewis Structures Of Interhalogen Compounds.
Lewis structures are a graphical representation of the arrangement of atoms and bonds in a molecule. They show the distribution of valence electrons, which are the electrons in the outermost shell of an atom that participate in chemical bonding. Interhalogen compounds are molecules that contain two or more different halogens.
To draw the Lewis structure of an interhalogen compound, first, determine the total number of valence electrons in the molecule. Then, connect the atoms with single bonds and distribute the remaining electrons as lone pairs on the atoms.
For example, the Lewis structure of chlorine trifluoride (ClF3) is:

In this structure, the chlorine atom has seven valence electrons, and each fluorine atom has seven valence electrons. The total number of valence electrons is 21. The chlorine atom is bonded to each fluorine atom by a single bond, and each fluorine atom has three lone pairs of electrons.
Lone Pair Addition, Add lone pairs to these lewis structures of interhalogen compounds.
In some cases, it is necessary to add lone pairs to the Lewis structure of an interhalogen compound in order to satisfy the octet rule. The octet rule states that each atom in a molecule should be surrounded by eight valence electrons.
For example, the Lewis structure of iodine monochloride (ICl) is:

In this structure, the iodine atom has seven valence electrons, and the chlorine atom has seven valence electrons. The total number of valence electrons is 14. The iodine atom is bonded to the chlorine atom by a single bond, but the iodine atom has only six valence electrons around it.
To satisfy the octet rule, we can add a lone pair of electrons to the iodine atom:

Resonance Structures
In some cases, it is necessary to use resonance structures to represent an interhalogen compound. Resonance structures are two or more Lewis structures that have the same number of valence electrons and the same arrangement of atoms, but they differ in the placement of double bonds and lone pairs.
For example, the Lewis structure of sulfur tetrafluoride (SF4) can be represented by two resonance structures:

In the first resonance structure, the sulfur atom has four single bonds to the fluorine atoms. In the second resonance structure, the sulfur atom has one double bond to one of the fluorine atoms and two single bonds to the other three fluorine atoms.
Both resonance structures satisfy the octet rule for all of the atoms in the molecule.
Examples
The following table provides a list of examples of interhalogen compounds, their Lewis structures, and the lone pairs that have been added:
| Interhalogen Compound | Lewis Structure | Lone Pairs Added |
|---|---|---|
| Chlorine trifluoride |  |
0 |
| Iodine monochloride |  |
1 |
| Sulfur tetrafluoride |  |
0 |
| Bromine pentafluoride |  |
1 |
| Iodine heptafluoride |  |
0 |
Essential FAQs
Why is adding lone pairs to Lewis structures important?
Adding lone pairs ensures that the octet rule is satisfied, which is a fundamental principle in chemistry. It helps us accurately represent the electronic configuration and bonding behavior of interhalogen compounds.
How do we determine the number of lone pairs to add?
The number of lone pairs added is determined by the number of valence electrons in the interhalogen compound. Each halogen atom contributes its valence electrons, and we add lone pairs to satisfy the octet rule for each atom.
What is the significance of resonance structures in representing interhalogen compounds?
Resonance structures are important for interhalogen compounds because they can have multiple Lewis structures that contribute to their overall bonding. Resonance structures help us understand the delocalization of electrons and the stability of different electronic configurations.