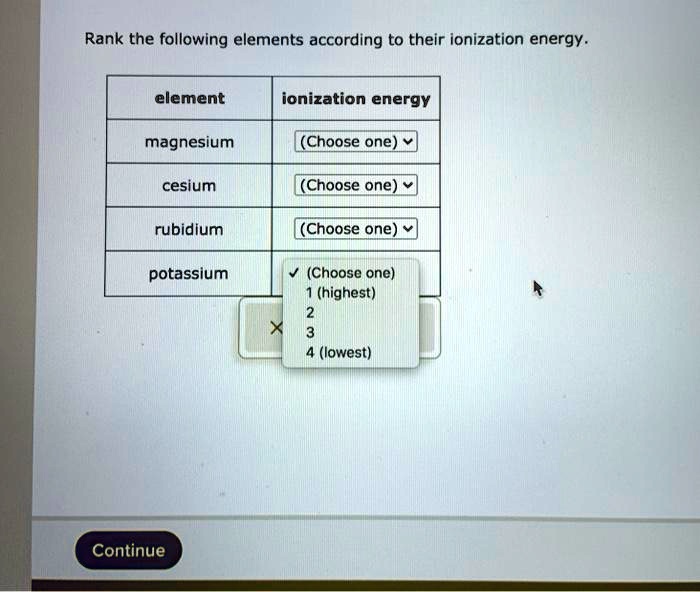
Rank the following elements according to their ionization energy sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. This in-depth guide delves into the intricacies of ionization energy, unraveling its definition, exploring its influential factors, and showcasing its practical applications across diverse scientific disciplines.
Delve into the captivating world of chemistry as we embark on a journey to unravel the mysteries of ionization energy, a fundamental concept that governs the behavior of elements and shapes the very fabric of our universe.
Ionization Energy: Definition and Concept
Ionization energy refers to the minimum energy required to remove an electron from an atom or ion in its gaseous state. It is a crucial concept in chemistry as it provides insights into the chemical reactivity and stability of elements.
Examples of ionization energy include:
- The first ionization energy of sodium (Na) is 496 kJ/mol, which is the energy needed to remove one electron from a sodium atom.
- The second ionization energy of magnesium (Mg) is 1451 kJ/mol, which is the energy required to remove a second electron from a magnesium ion with a +1 charge.
Several factors influence ionization energy, including:
- Atomic radius: Larger atoms have lower ionization energies due to the increased distance between the nucleus and the outermost electron.
- Nuclear charge: Elements with higher atomic numbers have more protons in their nuclei, resulting in a stronger attraction for electrons and higher ionization energies.
- Electron configuration: The number and arrangement of electrons in an atom’s orbitals affect its ionization energy.
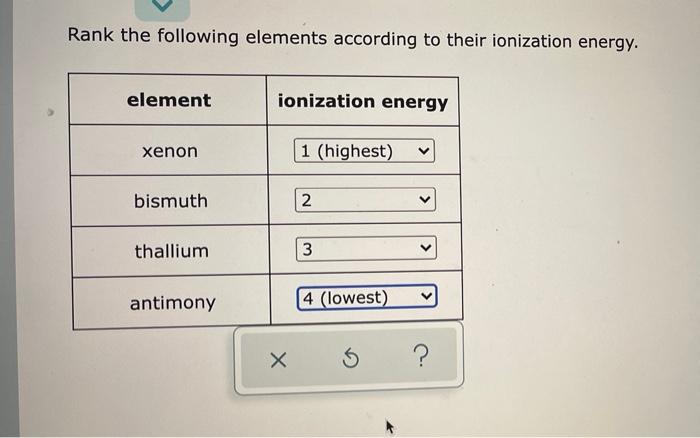
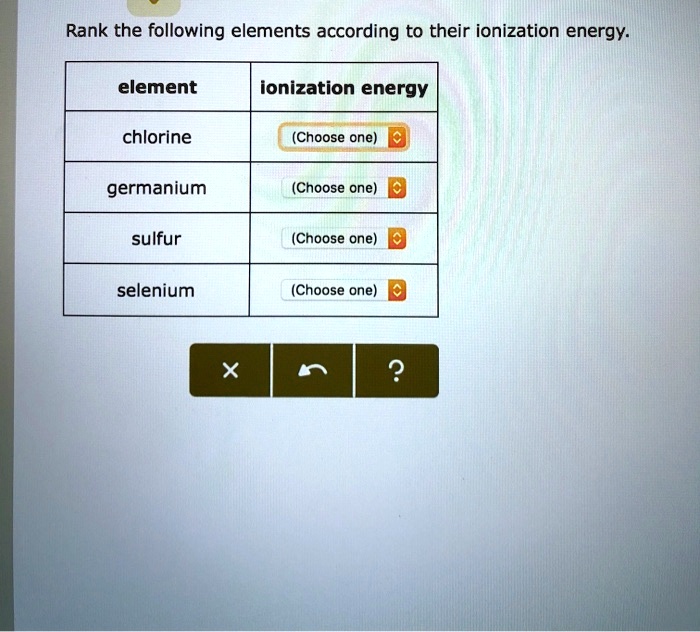
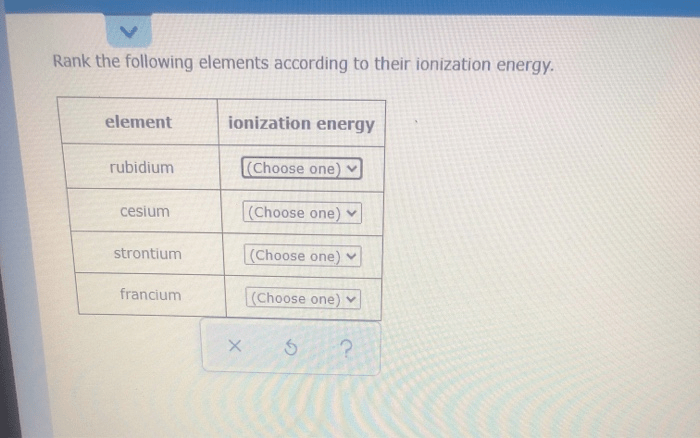
Ranking Elements Based on Ionization Energy
| Element | First Ionization Energy (kJ/mol) | Second Ionization Energy (kJ/mol) | Third Ionization Energy (kJ/mol) |
|---|---|---|---|
| Hydrogen (H) | 1312 | 3200 | 6000 |
| Helium (He) | 2372 | 5250 | 7500 |
| Lithium (Li) | 520 | 7300 | 11800 |
| Beryllium (Be) | 899 | 1757 | 14850 |
| Boron (B) | 801 | 2427 | 3650 |
The ranking system is based on the first ionization energy of the elements. Elements with lower ionization energies are more likely to lose electrons and are considered more reactive.
Factors Affecting Ionization Energy: Rank The Following Elements According To Their Ionization Energy
- Atomic radius:As atomic radius increases, ionization energy decreases. This is because the outermost electron is further away from the nucleus, experiencing less attraction and requiring less energy to remove.
- Nuclear charge:Ionization energy increases with increasing nuclear charge. This is because the increased number of protons in the nucleus creates a stronger electrostatic attraction for the electrons, making it more difficult to remove them.
- Electron configuration:The electron configuration of an atom can affect its ionization energy. Atoms with stable electron configurations, such as noble gases, have higher ionization energies because removing an electron would disrupt their stability.
Applications of Ionization Energy Data
- Predicting chemical reactivity:Ionization energy can help predict the reactivity of elements. Elements with low ionization energies are more likely to react with other elements, forming ionic bonds.
- Understanding periodic trends:Ionization energy follows periodic trends, with elements in the same group having similar ionization energies. This pattern can help understand the chemical properties of elements.
- Applications in various fields:Ionization energy data is used in fields such as plasma physics, astrophysics, and nuclear chemistry to study the behavior of atoms and ions in different environments.
Common Queries
What is ionization energy?
Ionization energy refers to the minimum amount of energy required to remove an electron from an atom or ion in its gaseous state.
Which factors influence ionization energy?
Ionization energy is primarily influenced by the atomic radius, nuclear charge, and electron configuration of an element.
How is ionization energy data used in chemistry?
Ionization energy data finds applications in predicting chemical reactivity, understanding periodic trends, and contributing to advancements in various fields of science and technology.