Calculate the number of molecules in 5.00 moles h2s – Calculate the number of molecules in 5.00 moles of H2S. The mole is a unit of measurement for chemical substances, and Avogadro’s number is a constant that represents the number of atoms or molecules in one mole of a substance.
Using these concepts, we can determine the number of molecules in a given sample of H2S.
H2S is a colorless gas with a strong odor. It is highly toxic and can be fatal if inhaled in high concentrations. H2S is used in a variety of industrial processes, including the production of sulfuric acid and the removal of sulfur from natural gas.
Introduction: Calculate The Number Of Molecules In 5.00 Moles H2s
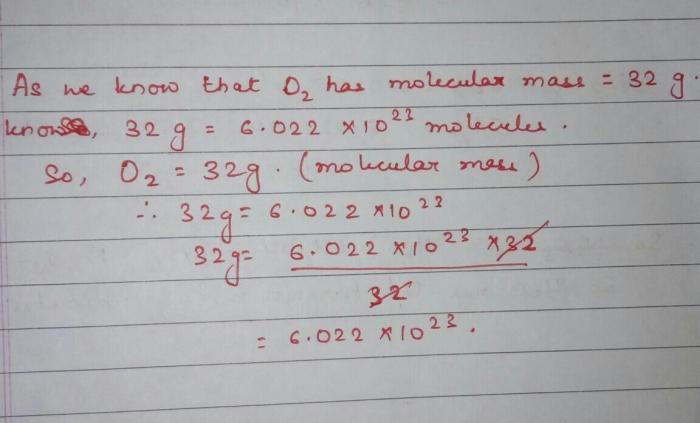
The mole is a fundamental unit of measurement in chemistry, representing a specific quantity of a substance. It is defined as the amount of a substance that contains exactly 6.02214076 x 10^23 elementary entities (atoms, molecules, ions, or electrons).
Hydrogen sulfide (H2S) is a colorless, flammable, and toxic gas with a distinctive rotten egg odor. It is a common pollutant in industrial areas and can also be found naturally in volcanic gases and certain types of bacteria.
Calculation of Number of Molecules
To calculate the number of molecules in a given number of moles, we use the following formula:
Number of molecules = Number of moles x Avogadro’s number
Avogadro’s number, denoted as N A, is a constant that represents the number of elementary entities present in one mole of a substance. Its value is 6.02214076 x 10^23.
Given 5.00 moles of H2S, we can calculate the number of molecules as follows:
Number of molecules = 5.00 moles x 6.02214076 x 10^23 molecules/mole = 3.011 x 10^24 molecules
Elaboration on Avogadro’s Number, Calculate the number of molecules in 5.00 moles h2s
Avogadro’s number was first proposed by the Italian scientist Amedeo Avogadro in 1811. It played a crucial role in determining the atomic mass of elements and establishing the concept of molecular weight.
Avogadro’s number is used in various fields of chemistry, including:
- Determining the molar mass of compounds
- Calculating the concentration of solutions
- Predicting the behavior of chemical reactions
Applications of the Calculation

Calculating the number of molecules in a given sample has practical applications in various scientific disciplines:
- Chemistry:Determining the concentration of solutions, predicting the behavior of chemical reactions, and understanding the stoichiometry of reactions.
- Biology:Estimating the number of cells in a sample, determining the concentration of biomolecules, and studying the kinetics of biochemical reactions.
- Materials Science:Characterizing the structure and properties of materials, such as the number of atoms or molecules in a crystal lattice.
Limitations and Considerations
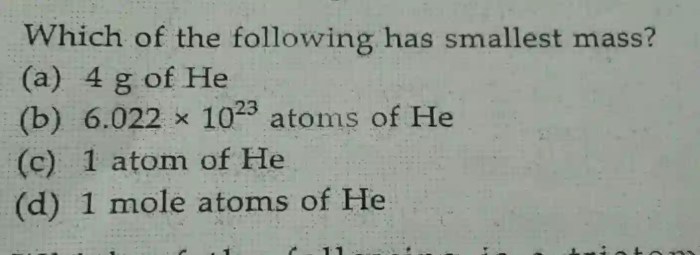
The formula for calculating the number of molecules assumes ideal behavior and the absence of impurities. In reality, deviations from ideal behavior can occur, leading to errors in the calculation.
Factors that can affect the accuracy of the calculation include:
- Non-ideal behavior of gases at high pressures or low temperatures
- Presence of impurities or contaminants
- Experimental errors in measuring the mass or volume of the sample
To minimize errors, it is important to use accurate measurement techniques, consider the non-ideality of the gas, and account for the presence of impurities when possible.
FAQ Overview
What is the mole concept?
The mole is a unit of measurement for chemical substances. It is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
What is Avogadro’s number?
Avogadro’s number is a constant that represents the number of atoms or molecules in one mole of a substance. It is equal to 6.022 × 10^23.
How can I calculate the number of molecules in a given sample?
To calculate the number of molecules in a given sample, you can use the following formula: Number of molecules = Number of moles × Avogadro’s number.