Welcome to the realm of chemical reactions, where the “Types of Reactions Worksheet Key” serves as your ultimate guide. This comprehensive resource delves into the fascinating world of chemical transformations, empowering you with a thorough understanding of the mechanisms, applications, and significance of these reactions in our daily lives.
As we embark on this journey, we will explore the diverse types of reactions, unraveling their intricacies through real-world examples. We will decipher the mechanisms that govern these reactions, examining the roles of reactants, products, and catalysts. Furthermore, we will delve into the practical applications of these reactions, showcasing their impact on various fields and industries.
Types of Reactions: Types Of Reactions Worksheet Key
Chemical reactions are processes that involve the rearrangement of atoms and molecules. There are several different types of reactions, each with its own unique characteristics and applications.
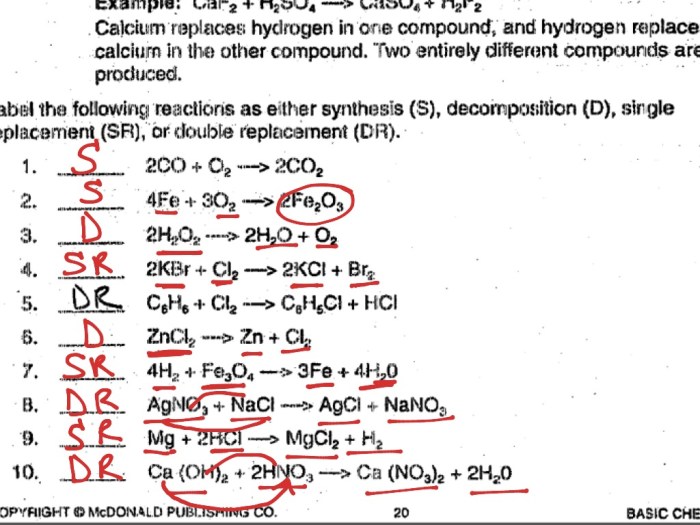
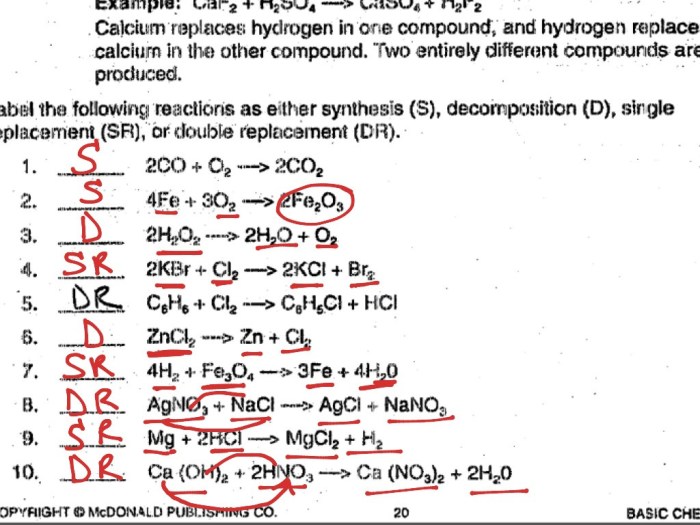
Combination Reactions, Types of reactions worksheet key
Combination reactions occur when two or more substances combine to form a single product. For example, hydrogen and oxygen combine to form water:
- 2H 2+ O 2→ 2H 2O
Decomposition Reactions
Decomposition reactions occur when a single substance breaks down into two or more products. For example, water can decompose into hydrogen and oxygen:
- 2H 2O → 2H 2+ O 2
Single-Replacement Reactions
Single-replacement reactions occur when one element replaces another element in a compound. For example, iron can replace copper in copper sulfate:
- Fe + CuSO 4→ FeSO 4+ Cu
Double-Replacement Reactions
Double-replacement reactions occur when the positive and negative ions of two compounds exchange places. For example, sodium chloride and silver nitrate can react to form sodium nitrate and silver chloride:
- NaCl + AgNO 3→ NaNO 3+ AgCl
Combustion Reactions
Combustion reactions occur when a substance reacts with oxygen to produce heat and light. For example, methane burns in oxygen to produce carbon dioxide and water:
- CH 4+ 2O 2→ CO 2+ 2H 2O
Worksheet Key
| Type of Reaction | Key |
|---|---|
| Combination | Two or more substances combine to form a single product. |
| Decomposition | A single substance breaks down into two or more products. |
| Single-Replacement | One element replaces another element in a compound. |
| Double-Replacement | The positive and negative ions of two compounds exchange places. |
| Combustion | A substance reacts with oxygen to produce heat and light. |
Reaction Mechanisms
Reaction mechanisms describe the step-by-step process by which reactants are converted into products. The mechanisms of different types of reactions vary depending on the nature of the reactants and the reaction conditions.
In general, reactions involve the breaking and forming of chemical bonds. Reactants are the starting materials of a reaction, and products are the final products. Catalysts are substances that speed up the rate of a reaction without being consumed in the process.
Applications of Reactions
Chemical reactions are used in a wide variety of applications, including:
- Manufacturing of chemicals, pharmaceuticals, and materials
- Energy production
- Food processing
- Environmental remediation
- Medical diagnosis and treatment
For example, the combustion of fossil fuels is used to generate electricity, and the Haber process is used to produce ammonia for fertilizer.
Balancing Equations
Chemical equations are mathematical representations of chemical reactions. Balancing equations ensures that the number of atoms of each element is the same on both sides of the equation.
To balance an equation, coefficients are added to the reactants and products. Coefficients represent the number of molecules or moles of each substance involved in the reaction.
The following steps can be used to balance an equation:
- Write the unbalanced equation.
- Count the number of atoms of each element on both sides of the equation.
- Adjust the coefficients to make the number of atoms of each element the same on both sides.
- Check that the equation is balanced.
Question & Answer Hub
What are the different types of chemical reactions?
Chemical reactions can be classified into various types based on their characteristics, including combination, decomposition, single displacement, double displacement, and redox reactions.
How do I balance chemical equations?
Balancing chemical equations involves adjusting the coefficients in front of the chemical formulas to ensure that the number of atoms of each element is equal on both sides of the equation.
What is the role of a catalyst in a chemical reaction?
A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process.